
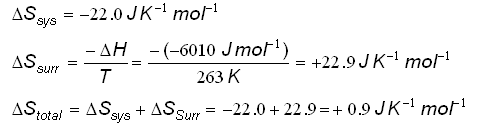
So what does "feasible" mean in reaction terms?Ī feasible reaction is one that is possible in terms of energy, but it doesn't mean that it will necessarily happen. If, for example, the entropy change of the reaction (the system) was +112 J K -1 mol -1, then the total entropy change would beįor a reaction to be feasible, the total entropy has to increase - in other words the sign of the total entropy change must be positive. An exothermic change heats the surroundings, and increases the entropy of the surroundings. Notice that the negative sign in the equation converts the negative exothermic enthalpy change into a positive entropy change. So if, say, you have an enthalpy change of -92.2 kJ mol -1, the value you must put into the equation is -92200 J mol -1. That means that if you are calculating entropy change, you must multiply the enthalpy change value by 1000. But entropy change is quoted in energy units of J. When you quote figures for enthalpy change they will have energy units of kJ. There is a mismatch between the units of enthalpy change and entropy change. That seems easy, but there is a major trap to fall in here, and if you manage to get through your course without falling into it at least once, you will have done really well! ΔH is the enthalpy change for the reaction. There is a simple equation for the entropy change of the surroundings.
#Entropy change formula how to#
So far, you know how to work out the entropy change of the system for a given reaction if you are told the entropies of all the substances involved in the reaction. You should be able to judge what is acceptable to your examiners by looking at how this is presented in your syllabus.Ĭalculating the entropy change of the surroundings. That is because we shall be using this equation under non-standard conditions. Note: I have deliberately left out the "standard" symbols in this equation. What matters is the total entropy change, which is the sum of the entropy changes of the system and the surroundings. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. The reverse is true for an endothermic change. And so increasing the temperature increases the entropy of the surroundings. If you add more energy to the surroundings, the number of different possibilities for arranging the energy over the molecules increases. Heat is given off to the surroundings, and that extra heat increases the entropy of the surroundings. If you only calculate the entropy change of the reaction (the entropy change of the system), you are leaving out an important factor. If you do need to read this page, make sure you have read the page explaining how you calculate the entropy change of the system first.

#Entropy change formula free#
You will still probably have to be able to work out the feasibility of reactions, but that will be done by the rather less confusing use of an equation based on Gibbs free energy. Note: If your syllabus doesn't specifically mention entropy change terms like system, surroundings and total, you could safely ignore this page.

It goes on to look at how you can use the total entropy change to decide whether or not a reaction is feasible. This page considers various entropy changes: of the system, of the surroundings, and the total change.


 0 kommentar(er)
0 kommentar(er)
